How to Conduct Effective Medical Device Design Reviews
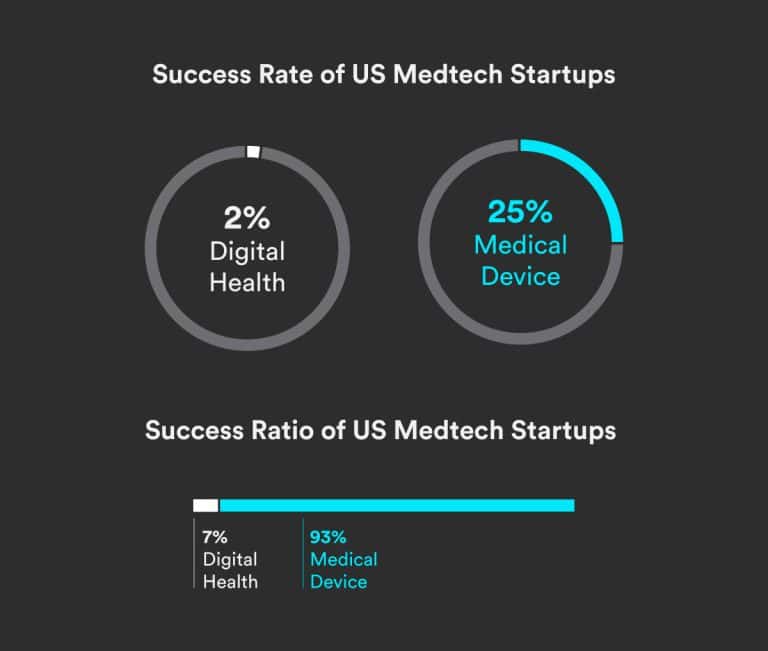
Design reviews are a mandatory part of medical device development and are often mistakenly treated as a compliance checkbox. When done properly, however, they can become a meaningful competitive advantage. This article explains why medical device design reviews matter and how to conduct them effectively—from what a design review is and who should be involved,…