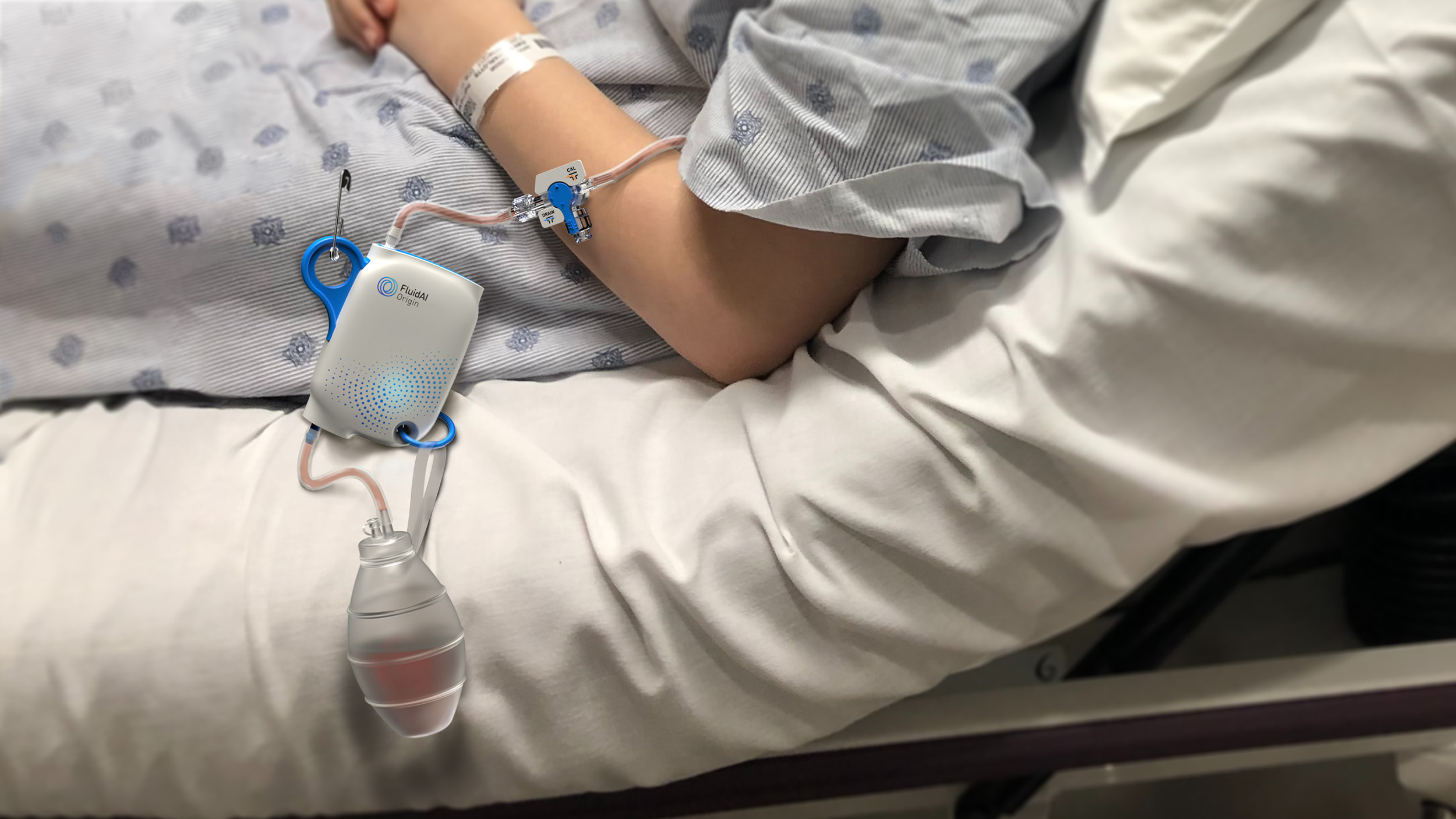
Building a Medical Device is Hard. We’re here to help.
Cortex Design supports bring your new medical technology to market by tackling user, technical, and regulatory challenges through our design-led process, guiding you as a trusted partner from concept to commercialization.
We are a certified ISO 13485:2016 Medical Product Development Firm.
Cortex Design specializes in design-led end-to-end development of medical devices and consumer health products. We craft products that elevate the human experience and enhance product value. Our team’s unique ability to uphold design vision throughout the regulated landscape of medical device development leads to product solutions that delight and inspire
We Excel At _____________.
- Product Strategy & Roadmapping
- Ethnographic Research | Usability
- Industrial Design
- UI/UX Design
- Mechanical Engineering
- Firmware & Electronics Development
- Prototyping
- Safety, Electrical, & Environmental Product Testing
- Design for Manufacturing & Assembly
- Contract Assembly & Production Coordination
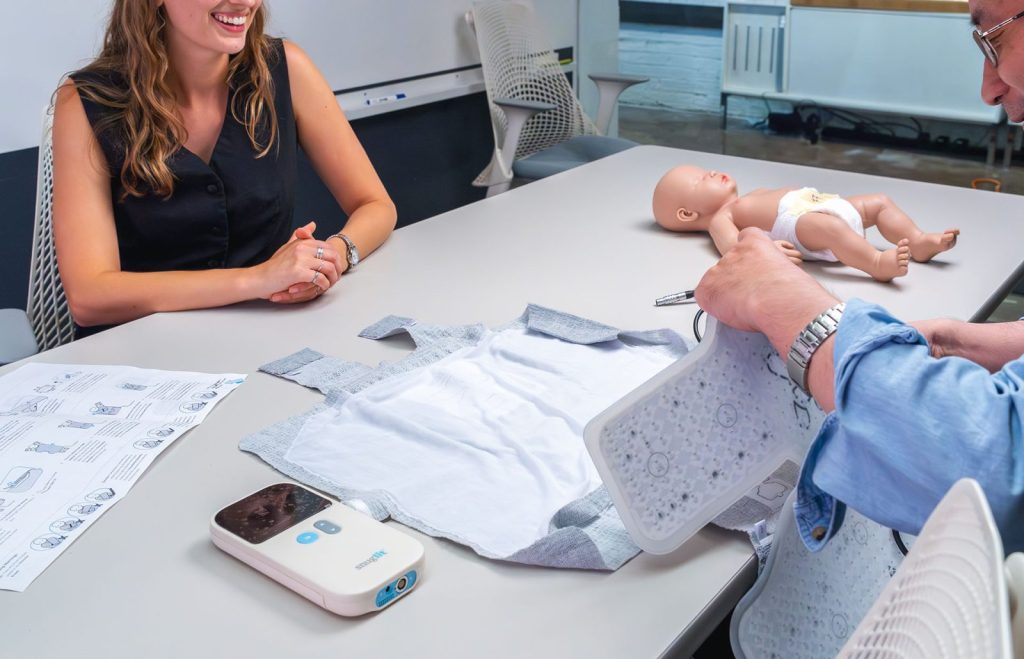

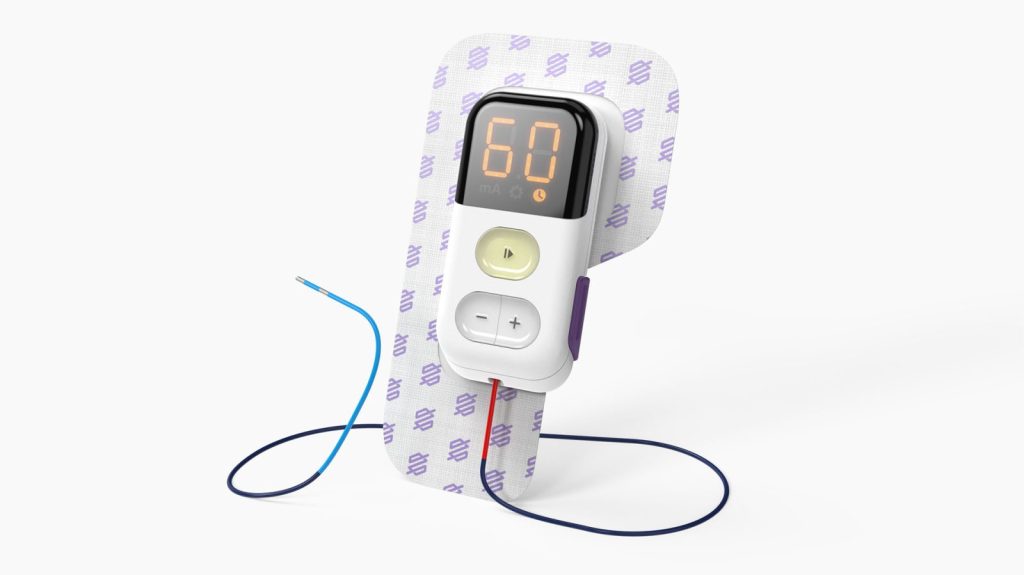
Medical Devices are Consumer Devices.
All products should delight. We meld the design thinking of consumer products with the rigour of medical device development. We consider all stakeholders when designing a product, balancing requirements of go-to-market cost, reimbursement, product architecture, and regulatory strategy/requirements. Our depth of in-house product development experience is supported by a trusted network of advisors who help clarify go-to-market challenges of medical devices.
Design Controls. By Designers. For Designers.
Bringing a new medical device to market is the opposite of user-centered design. We questioned the status quo and built a proprietary ISO 13485:2016 Quality Management System that accelerates medical device time to market. Conceptual design drives requirements in early project stages, then requirements drive engineering, prototyping and testing as the product is fully commercialized. This flexibility allows us to uncover novel solutions in early product phases and brings formality to build all design control documentation required for market submission.


Our Design Outputs. Your Submission.
A new medical device is more than just a new product, it’s also testing & documentation that proves to regulatory bodies that the device has been verified and validated, meets user needs and provides safe and effective treatment. As we develop your device, we build the required Design History File, or DHF, that is critical to your product’s successful market authorization.

Ready to take the next step?
Advance your Medical Device with Cortex Design.
Patients are demanding more of their healthcare experience- we help your product meet that demand.